Inventorying components, analyzing threats, managing requirements and evaluating residual risks in alignment with regulatory requirements and expectations
Engineers, designers and developers need to spend hours trying to decipher industry standards and write cybersecurity requirements. Our product cybersecurity experts and engineers have done this for you, using our own proprietary workflow and process.
Every team doesn’t have a cybersecurity expert, ProdSecDesigner demystifies the process and walks you through the work that has to get done.

Meet FDA and Regulatory Agency Requirements with ProdSecDesigner. The Omnibus bill signed into law that takes effect in October 2023, requires premarket submission for a cyber device to include information demonstrating that the cyber device meets cybersecurity requirements.
ProdSecDesigner enables you to work from an established baseline of cybersecurity requirements and manage residual risks throughout the total-product-lifecycle, helping to ensure a device is designed securely and kept that way.
Use our proven proprietary process and approach to add products, inventory components, evaluate threats and requirements, and manage residual risks. Use the output to support your regulatory submission.
Design Updates. Simplified. ProdSecDesigner enables
you to easily update your design and the corresponding
cybersecurity elements without churning through spreadsheets
and hours of re-writing, eliminating human error and providing
significant time savings.
When Industry Standards and Technical Requirements Update, ProdSecDesigner has you covered. Our experts monitor the regulations and do the heavy lift of reviewing all new standards and requirements. Updates will be made available in ProdSecDesigner for your re-evaluation and update. Focus your time on what you do, while we constantly review industry updates.
With your product inventoried in ProdSecDesigner, a validated platform, you have your Source of Truth for regulatory submissions. Retain it in ProdSecDesigner or export to your management tool.

ProdSecDesigner enables engineers to integrate security seamlessly into the core of their device development process.
It provides a comprehensive platform for customizing and managing threats, requirements, and residual risks, sourced from expert-crafted catalogs.
With built-in features like the CVSS calculator, engineers can ensure effective risk management and compliance throughout the product lifecycle.

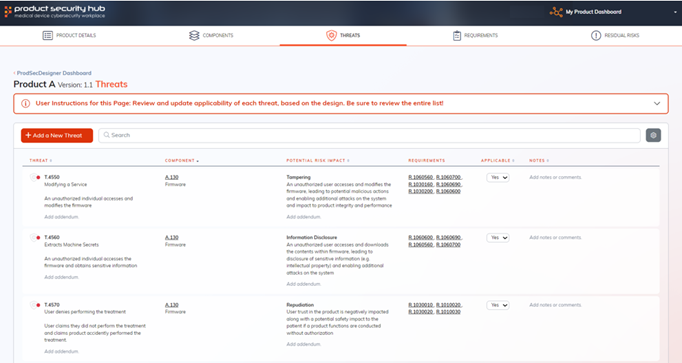
ProdSecDesigner streamlines the management of security requirements based on selected components from our proprietary catalog, which are pre-traced to industry standards.
You can also add new requirements as needed, ensuring comprehensive coverage.
In case of any unmet requirement, a residual risk will be generated, facilitating a robust risk management process throughout the product development cycle.
Easily capture and document residual risks arising from unimplemented security requirements or additional risks identified during activities such as penetration testing.
Detailed information about risk factors, severity, and potential mitigations can be added, empowering you to make informed decisions.
The built-in Common Vulnerability Scoring System (CVSS) calculator allows you to assess the severity of residual risks and prioritize mitigation efforts effectively.
ProdSecDesigner enables users to effortlessly create and manage SBOMs, helping meet both US FDA and the US National Telecommunications and Information Administration (NITA) minimum requirements.
The Vulnerability Management feature enables users to triage potential vulnerabilities, determine impact, and align them to threats, residual risks, and patches.
The Patch Tracking feature allows manufacturers to document cybersecurity patches and establish traceability to known residual risks in their products..